bi valence electrons|Valence Electrons : Tagatay Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical bonds. The number . 1080p Vixen Jia Lissa and Ellie Leen A Time And Place #Lesbian #Threesome #Beauty #Naturaltits #Hardcore #Cumshot #HighQuality #1080p #FullHD #MeetMyLove Backup / WATCH FULL HD 1080p on Link doodstream.com
PH0 · What Are Valence Electrons? Definition and Periodic Table
PH1 · What Are Valence Electrons? Definition and Periodic
PH2 · Valence electron
PH3 · Valence Electrons Chart for All Elements
PH4 · Valence Electrons
PH5 · Khan Academy
PH6 · Complete Electron Configuration of Bismuth (Bi, Bi3+, Bi5+)
PH7 · Complete Electron Configuration of Bismuth (Bi, Bi3+,
PH8 · Bismuth (Bi)
PH9 · Bismuth
PH10 · 11.1: Valence Electrons and the Periodic Table
PH11 · 10.6: Valence Electrons
Grand Rush Casino No Deposit Bonus Codes and Free Spins for August 2024. Bonus Info Code Wager Claim Bonus; 50 Free Spins. on Slots with exclusions: 50JOIN: 40x B: Claim Bonus: 70 Free Spins. on Peek Physique: 70PEEK: 50x B: Claim Bonus: $55 No Deposit Bonus. 55JOIN: 50x B: Claim Bonus: 333% up to $3330 match bonus. .
bi valence electrons*******Khan Academy Mar 23, 2023 Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical bonds. The number .In chemistry and physics, valence electrons are electrons in the outermost shell of an atom, and that can participate in the formation of a chemical bond if the outermost shell is not closed. In a single covalent bond, a shared pair forms with both atoms in the bond each contributing one valence electron. The presence of valence electrons can determine the element's chemical prope. Explain the relationship between the chemical behavior of families in the periodic table and their valence electrons. Identify elements that will have the most similar properties to a given element. The chemical properties of .
bi valence electrons This electron configuration shows that the bismuth ion(Bi 5+) has five shells and the last shell has eighteen electrons and it achieves a stable electron configuration. Bismuth atom exhibit +3, +5 oxidation states.
Bismuth is a chemical element of the periodic table with chemical symbol Bi and atomic number 83 with an atomic weight of 208.98 u and is classed as a post-transition metal. For example, alkali metal atoms (e.g., lithium, sodium) have one valence electron. Alkaline earth atoms (e.g., magnesium, calcium) have two valence electrons. The noble gases have complete octets, so all eight of .
Valence electrons are the electrons present in the outermost shell of an atom. You can easily determine the number of valence electrons an atom can have by looking at its Group in the .Element Bismuth (Bi), Group 15, Atomic Number 83, p-block, Mass 208.980. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. Jump to main content Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical bonds. The number of valence electrons in one atom of each element is easily determined based on its position in the periodic table. For the main group elements (groups designated with a . Bismuth is a classified post-transition metal and its symbol is ‘Bi’. Bismuth is the 83rd element of the periodic table so its atomic number is 83. The atomic number of an element is equal to the number of protons and electrons . This table of element valences includes the maximum valence and most common valence values in chemistry. Use this for reference with a periodic table. . While these are the most common valences, the real behavior of electrons is less simple. Remember an element's electron cloud will become more stable by filling, emptying, or half-filling the .bi valence electrons Valence Electrons The Modern Periodic Table and Chemical Bonds. As described in Section 10.6, the modern periodic table is arranged based on an atom's valence electrons.But what does this tell us about how they form chemical bonds with each other? Why do sodium atoms and chlorine atoms combine in a 1:1 ratio, while sodium and oxygen atoms combine in a 2:1 ratio?Valence electrons are the electrons present in the outermost shell of an atom. You can easily determine the number of valence electrons an atom can have by looking at its Group in the periodic table. For example, atoms in Groups 1 and 2 have 1 and 2 valence electrons, respectively. Atoms in Groups 13 and 18 have 3 and 8 valence electrons .A valence electron is an electron that is associated with an atom, and that can participate in the formation of a chemical bond; in a single covalent bond, both atoms in the bond contribute one valence electron in order to form a shared pair. The presence of valence electrons can determine the element's chemical properties and whether it may .
How many valence electrons does the element Bi have, and what are the specific valence electrons for Bi? Ground-state Electron Configuration: The ground-state electron configuration is the electron configuration of a neutral atom i.e. the number of electrons equals the number of protons. The ground-state configuration of an atom is related to .Valence electrons in the phosphate ion are 32. Example Problem 2 - Calculating Valence Electrons in a Molecule or Polyatomic Ion. Calculate the number of valence electrons in the ammonium ion {eq . In order to find the valence electrons of Bismuth atom (Bi), you can use two methods. Method 1: From the Periodic Table. To find out the valence electrons of Bismuth, you have to see the position of bismuth in the periodic table.
The chlorine atom has the same electron configuration in the valence shell, but because the entering electron is going into the n = 3 shell, it occupies a considerably larger region of space and the electron–electron repulsions are reduced. The entering electron does not experience as much repulsion and the chlorine atom accepts an additional .
Valence Electrons In order to find the valence electrons of Bismuth atom (Bi), you can use two methods. Method 1: From the Periodic Table. To find out the valence electrons of Bismuth, you have to see the position of bismuth in the periodic table. The chlorine atom has the same electron configuration in the valence shell, but because the entering electron is going into the n = 3 shell, it occupies a considerably larger region of space and the electron–electron repulsions are reduced. The entering electron does not experience as much repulsion and the chlorine atom accepts an additional .
Members of a group typically have similar properties and electron configurations in their outer shell. Period A horizontal row in the periodic table. The atomic number of each element increases by one, reading from left to right. . 209 Bi Electron configuration [Xe] 4f 1 4 5d 1 0 6s 2 6p 3 CAS number: 7440-69-9 ChemSpider ID:
Electron configuration of Bismuth (Bi) [Xe] 4f 14 5d 10 6s 2 6p 3: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 6 4f 14 5d 10 6s 2 6p 3: 2, 8, 18, 32, 18, 5: 84: . Periodic table with Valence Electrons Labeled (7 HD Images) Periodic table with Charges Labeled on it (7 HD Images) Electronegativity Chart of All Elements (All Values .Trimethylarsine, with a lone pair of electrons on the arsenic atom, can act as either a Lewis base or a reductant. If arsenic is oxidized by two electrons, then oxygen must be reduced, most probably by two electrons to the −2 oxidation state. Because As(V) forms strong bonds to oxygen due to π bonding, the expected product is (CH 3) 3 As=O. Find your element on the table. Now, locate the element that you want to find the valence electrons for on the table. You can do this with its chemical symbol (the letters in each box), its atomic number (the number in the top left of each box), or any of the other pieces of information available to you on the table.. For example purposes, let's find the valence .
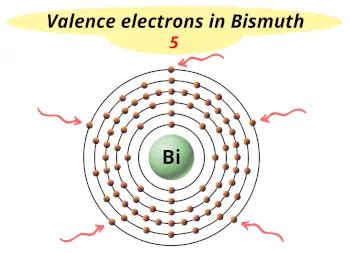
The valence electrons, electrons in the outermost shell, are the determining factor for the unique chemistry of the element. . (symbolized Bi, with Z = 83). The periodic table gives the following electron configuration: 1s 2 2s 2 2p 6 3s 2 3p .

Lithium has a single electron in the second principal energy level, and so we say that lithium has one valence electron. Li: 1s 2 2s 1 (the electron in the 2s energy level is the valence electron) Beryllium has two valence electrons: Be: 1s 2 2s 2 (the two electrons in the 2s energy level are the valence electrons)
See Pinaypie's newest porn videos and official profile, only on Pornhub. Visit us every day because we have all the latest Pinaypie sex videos awaiting you. Pornhub's amateur model community is here to please your kinkiest fantasies.
bi valence electrons|Valence Electrons